In
chemistry, we use symbols to represent the various chemicals. Success in
chemistry depends upon developing a strong familiarity with these basic
symbols. For example, the symbol "C"represents an atom of carbon, and
"H" represents an atom of hydrogen. To represent a molecule of table
salt, sodium chloride, we would use the notation "NaCl", where
"Na" represents sodium and "Cl" represents chlorine. We
call chlorine "chloride" in this case because of its connection to
sodium. You should have reviewed naming schemes, or nomenclature, in earlier readings.
A chemical equation is an expression of a chemical process. For
example:
AgNO3(aq) + NaCl(aq) ---> AgCl(s) + NaNO3(aq)
In this equation, AgNO3 is mixed with NaCl. The
equation shows that the reactants (AgNO3 and
NaCl) react through some process (--->) to form the products (AgCl
and NaNO3). Since they undergo a chemical process, they are changed
fundamentally.
Often chemical
equations are written showing the state that
each substance is in. The (s) sign means that the compound is a solid. The (l)
sign means the substance is a liquid. The (aq) sign stands for aqueous in water
and means the compound is
dissolved in water. Finally, the (g) sign means that the compound is a gas.
Coefficients are used in all chemical equations to show the
relative amounts of each substance present. This amount can represent either
the relative number of molecules,
or the relative number of moles (described below). If no coefficient is shown,
a one (1) is assumed.
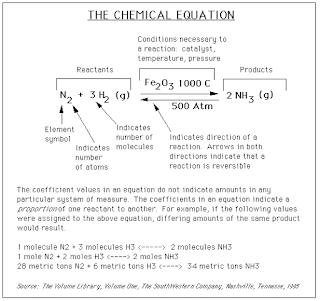
On some occasions, a variety of information will be written above
or below the arrows. This information, such as a value for temperature, shows
what conditions need to be present for a reaction to occur. For example, in the
graphic below, the notation above and below the arrows shows that we need a
chemical Fe2O3, a temperature of 1000° C, and a pressure
of 500 atmospheres for this reaction to occur. The graphic below works to
capture most of the concepts described above:
Sometimes,
however, we have to do some work before using the coefficients of the terms to represent the
relative number of molecules of each compound. This is the case when the
equations are not properly balanced. We will consider the following equation:
Al + Fe3O4 ---> Al2O3 +
Fe
Since no coefficients are in front of any of the terms, it is easy
to assume that one (1) mole of Al and one (1) mole of Fe3O4 react
to form one (1) mole of Al2O3. If this were the case, the
reaction would be quite spectacular: an aluminum atom would appear out of
nowhere, and two (2) iron atoms and one (1) oxygen atom would magically
disappear. We know from the Law of Conservation of Mass (which
states that matter can neither be created nor destroyed) that this simply
cannot occur. We have to make sure that the number of atoms of each
particular element in
the reactants equals the number of atoms of that same element in the products.
To do this we have to figure out the relative number of molecules of each term
expressed by the term's coefficient.
Balancing a simple chemical equation is essentially done by trial
and error. There are many different ways and systems of doing this, but for all
methods, it is important to know how to count the number of atoms in an
equation. For example we will look at the following term.
2Fe3O4
This term expresses two
(2) molecules of Fe3O4. In each molecule of this
substance there are three (3) Fe atoms. Therefore in two (2) molecules of the
substance there must be six (6) Fe atoms. Similarly there are four (4) oxygen
atoms in one (1) molecule of the substance so there must be eight (8) oxygen
atoms in two (2) molecules.
Now let's try balancing
the equation mentioned earlier:
Al + Fe3O4---> Al2O3+
Fe
Developing a strategy can be difficult, but here is one way of
approaching a problem like this.
1. Count the number of each atom on the reactant and on the product
side.
2. Determine a term to balance first. When looking at this problem,
it appears that the oxygen will be the most difficult to balance so we'll try
to balance the oxygen first. The simplest way to balance the oxygen terms is:
Al + 3 Fe3O4 --->
4 Al2O3+ Fe
Be sure to notice that the
subscript times the coefficient will give the number of atoms of that element.
On the reactant side, we have a coefficient of three (3) multiplied by a
subscript of four (4), giving 12 oxygen atoms. On the product side, we have a
coefficient of four (4) multiplied by a subscript of three (3), giving 12
oxygen atoms. Now, the oxygens are balanced.
3. Choose another term to balance. We'll choose iron, Fe. Since there
are nine (9) iron atoms in the term in which the oxygen is balanced we add a
nine (9) coefficient in front of the Fe. We now have:
Al +3 Fe3O4 --->
4Al2O3+ 9Fe
4. Balance
the last term. In this case, since we had eight (8) aluminum atoms on the
product side we need to have eight (8) on the reactant side so we add an eight
(8) in front of the Al term on the reactant side.
Now, we're done, and the balanced equation is:
8Al + 3Fe3O4 --->
4Al2O3 + 9 Fe
Sometimes when reactions occur between two or
more substances, one reactant runs out before the other. That is called the
"limiting reagent". Often, it is necessary to identify the limiting
reagent in a problem.
Example: A chemist only has 6.0 grams of C2H2 and an unlimited supply of oxygen and he desires to
produce as much CO2 as possible. If she uses the equation below, how much
oxygen should she add to the reaction?
2C2H2(g)
+ 5O2(g) ---> 4CO2(g) + 2 H2O(l)
To solve this problem, it is necessary to determine how much
oxygen should be added if all of the reactants were used up (this is the way to
produce the maximum amount of CO2).
First, we calculate the number of moles of C2H2 in
6.0 g of C2H2. To be able to calculate the moles we need
to look at a periodic table and see that 1 mole of C weighs 12.0 g and H weighs
1.0 g. Therefore we know that 1 mole of C2H2 weighs
26 g (2 × 12 grams + 2 × 1 gram).
6.0
g C2H2 x
|
1 mol C2H2
--------------------- (24.0 + 2.0)g C2H2 |
=
0.25 mol C2H2
|
Then, because there are
five (5) molecules of oxygen to every two (2) molecules of C2H2,
we need to multiply the result by 5/2 to get the total molecules of oxygen.
Then we convert to grams to find the amount of oxygen that needs to be added:
0.25
mol C2H2 x
|
5 mol O2
-------------- 2 mol C2H2 |
x
|
32.0 g O2
--------------- 1 mol O2 |
=
20 g O2
|
It is possible to calculate the mole ratios (also
called mole fractions) between terms in a chemical equation when given the
percent by mass of products or reactants.
percentage by mass = mass
of part/ mass of whole
There are two types of percent composition problems-- problems in which you are given the formula
(or the weight of each part) and asked to calculate the percentage of each
element and problems in which you are given the percentages and asked to
calculate the formula.
In percent composition
problems, there are many possible solutions. It is always possible to double
the answer. For example, CH and C2H2 have the same
proportions, but they are different compounds. It is standard to give compounds
in their simplest form, where the ratio between the elements is as reduced as
it can be-- called the empirical formula. When calculating the
empirical formula from percent composition, one can convert the percentages to
grams. For example, it is usually the easiest to assume you have 100 g so 54.3%
would become 54.3 g. Then we can convert the masses to moles; this gives us
mole ratios. It is necessary to reduce to whole numbers. A good technique is to
divide all the terms by the smallest number of moles. Then the ratio of the
moles can be transferred to write the empirical formula.
Example: If a compound is 47.3% C (carbon), 10.6% H (hydrogen) and
42.0% S (sulfur), what is its empirical formula?
To do this problem we need to transfer all of our percents to
masses. We assume that we have 100 g of this substance. Then we convert to moles:
Carbon:
|
47.3 grams
-------------------- 1 |
x
|
1 mole
----------------- 12.01 grams |
=
3.94 moles
|
Hyrdrogen:
|
10.6 grams
--------------- 1 |
x
|
1 mole
------------- 1.008 grams |
=
10.52 moles
|
Sulfur:
|
42.0 grams
-------------- 1 |
x
|
1 mole
---------------- 32.07 grams |
=
1.310 moles
|
Now we try to get an even
ratio between the elements so we divide by the number of moles of sulfur,
because it is the smallest number:
Carbon:
|
3.94
------ 1.310 |
=
3
|
Hydrogen:
|
10.52
------ 1.310 |
=
8
|
Sulfur:
|
1.310
------ 1.310 |
=
1
|
So we have: C3H8 S
Example: Figure out the percentage by mass of hydrogen sulfate, H2SO4.
In this problem we need to first calculate the total mass of the
compound by looking at the periodic table. This gives us:
2(1.008) + 32.07 + 4(16.00) g/mol = 98.09 g/mol
Now, we need to take the weight fraction of each element over the
total mass (which we just found) and multiply by 100 to get a percentage.
hydrogen:
|
2(1.008)
--------- 98.09 |
=
|
2.016
------ 98.09 |
=
0.0206 ∗ 100 = 2.06%
|
sulfur:
|
32.07
---------- 98.09 |
=
0.327 ∗ 100 = 32.7%
|
||
oxygen:
|
4(16.00)
--------- 98.09 |
=
|
64.00
------ 98.09 |
=
0.652 ∗ 100 = 65.2%
|
Now, we can check that the
percentages add up to 100%
65.2 + 2.06 + 32.7 = 99.96
This is essentially 100 so we know that
everything has worked, and we probably have not made any careless errors.
So the answer is that H2SO4 is made up of 2.06% H, 32.7% S, and 65.2% O by mass.
While the empirical formula is the simplest form
of a compound, the molecular formula is the form of the term as it would appear
in a chemical equation. The empirical formula and the molecular formula can be
the same, or the molecular formula can be any positive integer multiple of the
empirical formula. Examples of empirical formulas: AgBr, Na2S, C6H10O5. Examples of molecular formulas: P2, C2O4, C6H14S2, H2, C3H9.
One can calculate the
empirical formula from the masses or percentage composition of any compound. We
have already discussed percent composition in the section above. If we only
have mass, all we are doing is essentially eliminating the step of converting
from percentage to mass.
Example: Calculate the empirical formula for a compound that has 43.7
g P (phosphorus) and 56.3 grams of oxygen. First we convert to moles:
43.7 grams P
------------- 1 |
x
|
1 mol
----------- 30.97 grams |
=
1.41 moles
|
|
56.3 grams O
------------- 1 |
x
|
1 mol
-------------- 16.00 grams |
=
3.52 moles
|
|
Next we divide the moles to try to get an even ratio.
Phosphorus:
|
1.41
----- 1.41 |
=
1.00
|
Oxygen:
|
3.52
----- 1.41 |
=
2.50
|
When we divide, we did not
get whole numbers so we must multiply by two (2). The answer = P2O5
Calculating the molecular
formula once we have the empirical formula is easy. If we know the empirical
formula of a compound, all we need to do is divide the molecular mass of the
compound by the mass of the empirical formula. It is also possible to do this
with one of the elements in the formula; simply divide the mass of that element
in one mole of compound by the mass of that element in the empirical formula.
The result should always be a natural number.
Example: if we know that the empirical formula of a compound is
HCN and we are told that a 2.016 grams of hydrogen are necessary to make the
compound, what is the molecular formula? In the empirical formula hydrogen
weighs 1.008 grams. Dividing 2.016 by 1.008 we see that the amount of hydrogen
needed is twice as much. Therefore the empirical formula needs to be increased
by a factor of two (2). The answer is:
H2C2N2.
The concentration of a solution is the "strength" of a
solution. A solution typically refers to the dissolving of some solid substance
in a liquid, such as dissolving salt in water. It is also often necessary to
figure out how much water to add to a solution to change it to a specific concentration.
The concentration of a
solution is typically given in molarity. Molarity is
defined as the number of moles of solute (what is actually
dissolved in the solution) divided by the volume in liters of solution (the total volume of what
is dissolved and what it has been dissolved in).
Molarity
=
|
moles of solute
------------------ liters of solution |
Molarity is probably the
most commonly used term because measuring a volume of liquid is a fairly easy
thing to do.
Example: If 5.00 g of NaOH are dissolved in 5000 mL of water, what
is the molarity of the solution?
One of our first steps is to convert the amount of NaOH given in
grams into moles:
5.00g NaOH
-------------- 1 |
x
|
1 mole
------------------------- (22.9 + 16.00 + 1.008)g |
=
0.125 moles
|
Now we simply use the definition of molarity: moles/liters to get
the answer
Molarity
=
|
0.125 moles
--------------- 5.00 L of soln |
=
0.025 mol/L
|
So the molarity (M) of the solution is 0.025 mol/L.
Molality is another common
measurement of concentration. Molality is defined as moles of solute divided by
kilograms of solvent (the substance in which it
is dissolved, like water).
Molality
=
|
moles of solute
--------------- kg of solvent |
Molality is sometimes used
in place of molarity at extreme temperatures because the volume can contract or
expand.
Example: If the molality of a solution of C2H5OH dissolved in water is
1.5 and the mass of the water is 11.7 kg, figure out how much C2H5OH must have been added in
grams to the solution.
Our first step is to substitute what we know into the equation.
Then we try to solve for what we don't know: moles of solute. Once we know the
moles of solute we can look at the periodic table and figure out the conversion
from moles to grams.
|
Now we simply use the definition of molarity: moles/liters to get
the answer
|
|||||
|
|||||
|
|||||
|
It is possible to convert
between molarity and molality. The only information needed is density.
Example: If the molarity of a solution is 0.30 M, calculate the
molality of the solution knowing that the density is 3.25 g/mL.
To do this problem we can assume one (1) liter of solution to make
the numbers easier. We need to get from the molarity units of mol/L to the
molality units of mol/kg. We work the problem as follows, remembering that
there are 1000 mL in a Liter and 1000 grams in a kg. This conversion will only
be accurate at small molarities and molalities.
0.3 mol
---------- 1 L |
x
|
1 mL
------ 3.25 g |
x
|
1 L
-------------- 1000 mL |
x
|
1000 g
-------- 1 kg |
=
0.09 mols / kg
|
It is also possible to
calculate colligative properties,
such as boiling point depression, using molality. The equation for temperature
depression or expansion is
ΔT= Kf × m
Where:
ΔT is temperature depression (for freezing point) or
temperature expansion (for boiling point) (°C)
Kf is the freezing point
constant (kg °C/mol)
m is molality in mol/kg
Example: If the freezing point of the salt water put on roads is
-5.2° C, what is the molality of the solution? (The Kf for
water is 1.86 °C/m.)
This is a simple problem where we just plug in numbers into the
equation. One piece of information we do have to know is that water usually
freezes at 0° C.
how do you know when an equation is unbalanced?
BalasHapusWhen there are an unequal number of atoms between the two side. For example, having 3 Oxygen on one side and 2 on the other would be imbalanced.
Hapuswith the coefficients in front of reactants and products in equations, i always thought they represented the number of molecules of these substances. for example 2Na + Cl would mean 2 molecules of sodium reacting with one molecule of oxygen.
BalasHapushow can I reconcile that with the concept of stoichiometric coefficients, or is that completely wrong?
In your problem you put Chlorine gas but you said "reacts with oxygen" everythjng else in your problem is correct except for that piece. Let me put it into a problem. H2 + Cl2 = 2HCl because hydrogen and chlorine are diatomic so together they would form 2 molecules of HCl
HapusOne mole of H2SO4 reacts with 2 moles of NaOH.How does Avogadro"s number help to explain it?
BalasHapus1 molecule of H₂SO₄ + 2 formula units of NaOH → 1 formula unit of Na₂SO₄ + 2 molecules of H₂O
HapusMultiply both sides of the equation by Avogadro's number, N.
1N molecules of H₂SO₄ + 2N formula units of NaOH → 1N formula units of Na₂SO₄ + 2N molecules of H₂O
Avogadro's number is by definition the number of moles or formula units in 1 mol of any compound.
∴ 1 mol H₂SO₄ + 2 mol NaOH → 1 mol Na₂SO₄ + 2 mol H₂O
How to know the mole of a substance easily?
BalasHapusI think that you have to understand the basic concept of mole, that will easily know the mole of a substance.
HapusWhat do you mean by stoichiometry ?
BalasHapusThe word stoichiometry derives from two greek words that mean measuring the elements, stoichiometry deals with calculations about the masses and volumes of reactants and products insolved in a chemical reactions.
HapusWhat is the relationship of moles with mass and volume?
BalasHapusWe can find volume if we know that mass
HapusThe formula is mole = mass/Mr
and than to find volume in STP the formula is volume(STP) = mole x 22,4
Or to find volume is not in STP is
volume x P = mole x R x T
Give a question and its solution about the ideal gas ?
BalasHapus5.0 g of neon is at 256 mm Hg and at a temperature of 35º C. What is the volume?
HapusSOLUTION
Step 1: Write down your given information:
P = 256 mmHg
V = ?
m = 5.0 g
R = 0.0820574 L•atm•mol-1K-1
T = 35º C
Step 2: Convert as necessary:
Pressure: 256mmHg×(1atm/760mmHg)=0.3368atm
256
m
m
H
g
1
a
t
m
760
m
m
H
g
0.3368
a
t
m
Moles: 5.0gNe×(1mol/20.1797g)=0.25molNe
5.0
g
N
e
1
m
o
l
20.1797
g
0.25
m
o
l
N
e
Temperature: 35ºC+273=308K
35
C
273
308
K
Step 3: Plug in the variables into the appropriate equation.
V=(nRT/P)(1.12)
(1.12) VnRT
P
V=(0.25mol)(0.08206Latm/Kmol)(308K)(0.3368atm)](1.13)
(1.13) V
0.25
m
o
l
0.08206
L
a
t
m
K
m
o
l
308
K
0.3368
a
t
m
V=19
Can you explain about balancing simple chemical equations?
BalasHapusBalancing a simple chemical equation is essentially done by trial and error. There are many different ways and systems of doing this, but for all methods, it is important to know how to count the number of atoms in an equation. For example we will look at the following term.
Hapus2Fe3O4
This term expresses two (2) molecules of Fe3O4. In each molecule of this substance there are three (3) Fe atoms. Therefore in two (2) molecules of the substance there must be six (6) Fe atoms. Similarly there are four (4) oxygen atoms in one (1) molecule of the substance so there must be eight (8) oxygen atoms in two (2) molecules.
Now let's try balancing the equation mentioned earlier:
Al + Fe3O4---> Al2O3+ Fe
Developing a strategy can be difficult, but here is one way of approaching a problem like this.
1. Count the number of each atom on the reactant and on the product side.
2. Determine a term to balance first. When looking at this problem, it appears that the oxygen will be the most difficult to balance so we'll try to balance the oxygen first. The simplest way to balance the oxygen terms is:
Al + 3 Fe3O4 ---> 4 Al2O3+ Fe
Be sure to notice that the subscript times the coefficient will give the number of atoms of that element. On the reactant side, we have a coefficient of three (3) multiplied by a subscript of four (4), giving 12 oxygen atoms. On the product side, we have a coefficient of four (4) multiplied by a subscript of three (3), giving 12 oxygen atoms. Now, the oxygens are balanced.
3. Choose another term to balance. We'll choose iron, Fe. Since there are nine (9) iron atoms in the term in which the oxygen is balanced we add a nine (9) coefficient in front of the Fe. We now have:
Al +3 Fe3O4 ---> 4Al2O3+ 9Fe
4. Balance the last term. In this case, since we had eight (8) aluminum atoms on the product side we need to have eight (8) on the reactant side so we add an eight (8) in front of the Al term on the reactant side.
Now, we're done, and the balanced equation is:
8Al + 3Fe3O4 ---> 4Al2O3 + 9 Fe
we can use the stoichiometry in the reaction but What about that use in our life?
BalasHapusExplain the relationship between molarity and dilution?
BalasHapus