Corrosion
It’s
not necessary that all redox reactions are part of energy change. You can
notice many reactions around you which involve a negligible amount of energy or
occurs without change in energy. For example, Silver metal is oxidized when it
comes in contact in the atmosphere oxygen or hydrogen disulphide.
4Ag(s)+2H2S(g)+O2(g)→2Ag2S(s)+2H2O(g)4Ag(s)+2H2S(g)+O2(g)→2Ag2S(s)+2H2O(g)
The film of sulphide which collects on the metal surface acts as a protective
layer for further oxidation of metal surfaces. The tarnishing of silver is just
one example of a broad class of oxidation-reduction reactions that fall under
the general heading of corrosion. The rust of the
metals because of corrosion of the surface is an example of the oxidation
reaction.
Generally, metals are oxidized in contact with the air and covered by a layer
of metallic oxide. In aluminium metal, the cover of aluminium oxide is very
hard and thick which protects the metal surface against the later attack of the
oxygen of the air. You must have seen a rusty iron hammer, which corrode by
oxygen, but at a much slower rate than the burning wood. Do you know why it is
so? Let’s discuss some other points related to corrosion.
What is Corrosion
Corrosion is defined as "the
degradation of materials by chemical reaction with the environment in which the
material resides." This is because of metal
oxidation. As metals have a tendency to return to their natural state, it is a
natural process which produces either salt or oxides. It requires four elements
- anode, cathode, an electrolyte, and a metallic path.
Corrosion Material
Some
Points about Corrosion Materials has Given Below:
- They
are the materials which are the cause of corrosion.
- They
are toxic in nature.
- They
have very harmful effects as they have a tendency to attack metals and
destroy their strength.
- They
also affect the human body, especially tissues. Some acids and bases are
included in that.
- For
example, HCl, nitric and sulfuric acid and bases like sodium
hydroxide and ammonia.
Metal Corrosion
Metal
corrosion is the main cause of metal destruction, like steel rusts due to
immersion in seawater. Similarly iron reacts with oxygen to form rust by
exposure to moist air.
Iron rust is iron oxide Fe2O3.
XH2O where X is the amount of complexed water with ferric oxide,
which can vary. It shows the color of rust (black to yellow to orange).
Corrosion of Metal
It
is a very complex process which is completed in the following steps.
- Oxidation
of iron- First the iron gets oxidized into ferrous ions [Fe (II)]
with the loss of two electrons.
Fe → Fe+2+ 2 e-
- The
ferrous ions again get oxidized into ferric ions [Fe(III)] in the presence
of water and oxygen.
Fe+2 → Fe+3 + e-
- These
electrons from the above reactions are used to reduce oxygen.
O2(g) + 2 H2O
+ 4e- → 4 OH-
- The
ferric ions combine with oxygen and form ferric oxide [iron (III) oxide].
This ferric oxide gets hydrated with water.
The
complete chemical reaction for rust formation is shown below. The mechanism for
the rusting process is similar to the electrochemical cell. The electrons
formed during the oxidation of iron is conducted through the metal. Thus, the
iron ions diffuse from the water layer to the metal surface where oxygen is
present.
This is an electrochemical cell where iron acts as the anode and oxygen gas as the cathode. The aqueous solution of ions behaves like a
"salt bridge" as shown in the figure.
Rusting happens faster in the presence of
moisture rather than in a dry environment. This process is also affected by
some other factors like the presence of other salts, which increases the rate
of rusting, because the presence of salt enhances the conductivity of the
aqueous solution formed at the surface of the metal.
So the rusting of iron and steel is completed rapidly near the ocean (salty) or with salt.
Types of Corrosion
There
are different types of corrosion which depend on the environment surrounding
the material, type of material, chemical reaction etc. Some general types of
corrosion are described below.
1. Uniform Corrosion
This is also called General
corrosion. It is a very common method of corrosion. It deteriorates
the whole surface of the metal and makes the surface thin. The damage is done
at a constant rate on the entire surface. It can be easily detected by it's
appearance. It can be controlled but if it is not, it then destroys the whole
metal.
2. Galvanic Corrosion
This type of corrosion occurs with an electrolyte like seawater. Metals have
different values of electrical potentials. When they become electrically
connected and put in an electrolyte, the more active metal which has a high
negative potential becomes the anode. Due to it's high negative potential, it
corrodes fast. But the less active metal becomes the cathode.
The flow of electric current continues till the potentials are equal between both electrodes. So at the joint where the two non similar metals meet, the galvanic corrosion appears. The Galvanic Series shows the list of metals from the most active to the least active (most noble). Thus galvanic corrosion can be controlled by selecting the two metals which are close in series. As platinum is the least active, it is also less active for corrosion.
3. Pitting Corrosion
This occurs because of random attacks on particular parts of the metal's
surface. This makes holes which are large in depth. These holes are called
"pits". The pit acts as the anode while the undamaged part of the
metal is the cathode. It begins with a chemical breakdown in the form of a
scratch or spot. The pitting process makes the metal thinner and increases fatigue.
For example, it can be very harmful in gas lines.
4. Stress Corrosion Cracking (SCC)
It is a complex form of corrosion which arises due to stress and corrosive
environment. This generates brittle and dry cracks in the material. The brittle
cracks can inter or Trans granular morphology. The stress is developed in the
material due to bending or stretching of the material. It also affects only at
a particular section of material.
The main reasons for stress corrosion are welding, heating treatments,
deformation etc. It is very difficult to detect the cracks or detect stress
corrosion because they combine with active path corrosion. The active path
corrosion occurs generally along grain or crystallographic boundaries. Stress
corrosion is strongly affected by alloy
composition.
composition.
5. Corrosion fatigue
This occurs in the presence of a corrosive environment like saltwater. It is a
combination of cyclic stress and corrosion. Corrosion fatigue is produced when
a metal breaks at a stress level which is lower than its tensile strength. It
is strongly affected by the environment in which the metal resides which
affects the initiation and growth rate of the cracks. These cracks are too fine
to detect easily. So the stress coupons (metal sample) are used to detect the
corrosion.
It can be produced by the influence of various types of stress like stresses applied, thermal expansion, thermal contraction, welding, soldering, cleaning, heating treatment, construction process, casting etc. To prevent corrosion fatigue, the designing and construction process of the materials should be done properly, by eliminating any stress and environmental factors and by eliminating crevices.
It can be produced by the influence of various types of stress like stresses applied, thermal expansion, thermal contraction, welding, soldering, cleaning, heating treatment, construction process, casting etc. To prevent corrosion fatigue, the designing and construction process of the materials should be done properly, by eliminating any stress and environmental factors and by eliminating crevices.
6. Intergranular Corrosion
In the granular composition of metals and alloys, grains (small crystals) are
present and their surfaces join with each other. This forms the grain
boundaries. Thus the grains are separated by grain boundaries. Intergranular
corrosion is also known as inter crystalline corrosion. The Intergranular
corrosion is developed on or near the grain boundaries of a metal. This can be
due to welding, stress, heat treating or improper service etc. The metal can
loose its strength due to the Intergranular corrosion.
7. Crevice Corrosion
It is also known as concentration cell corrosion. This is due to the trapping
of liquid corrosive between the gaps of the metal. As the electrolyte has
aggressive ions like chlorides, the corrosion reaction is started after
settling of liquid in gaps. Oxygen is consumed during the reaction.
Thus an anodic area is developed near the oxygen-depleted zone while the external part of the material acts as a cathode. Crevice corrosion is similar to pitting corrosion. It’ very difficult to detect crevice corrosion. It can be initiated by materials like gaskets, fasteners, surface deposits, washers, threads, clamp etc.
8. Filiform corrosion
It is a type of concentration cell corrosion. This develops on coated metallic
surfaces with a thin organic film. The corrosion generates the defect on the
protective coating of metallic surface. The filaments of corrosion product is
the cause of degradation of the coating. The filaments look like thin threads.
They exist as long branching paths.
The actively growing filaments do not intersect the inactive filaments. The reflection process takes place when filaments collide with each other. Filiform corrosion is a very specific process because it only affects the surface’s appearance, not the metallic material.
9. Erosion Corrosion
It is also called flow-assisted corrosion. This is due to the movement of
corrosive liquids on metal surface which damages the material. It can be seen
in ship propellers which are constantly exposed to sea water or in soft alloys.
The damage can be seen as waves or rounded holes etc. It shows the flow of the
corrosive liquid. It can be controlled by the use of hard alloys, managing the
velocity and flow pattern of the fluid.
10. Fretting Corrosion
It is a form of erosion-corrosion. It shows as the combined effect of corrosion
and fretting of metal. Due to this corrosion, the material surface starts to
disappear. Fretting corrosion exists in the form of dislocations of the surface
and deep pits. Oxidation is the main cause of fretting corrosion. It can be
controlled by using lubricates, controlling movement etc.
11. Concentration Cell Corrosion
It occurs when two metal surfaces are in contact with different concentrations of the same solution.
Corrosion Theory
1. Water on the metal surface dissolves CO2 and O2 from the air.
2. Fe in contact with dissolved CO2 and O2 undergoes oxidation.
Fe → Fe2+ + 2e- - Anode
3. Electrons lost by Fe are taken by H+
H+ + e- → H
4H + O2
→ 2H2O4H + O2
On
multiplying the first equation by 4 and adding to the second,
4. Fe2+ reacts with dissolved O2 and water
Rust
(Hydrated ferric oxide)
Causes
of Corrosion
Given
below are some of the factors that cause corrosion.
- Reactivity
of metal
- Bacteria
- Presence
of impurities
- Presence
of air, moisture, gases like SO2 and CO2
- Presence
of electrolytes
Harmful Effects of Corrosion
Corrosion Protection
Two
methods are used for the protection of materials from corrosion.
- Cathodic
protection
- Corrosion
inhibitors.
Both
methods are based on charge control of the metal surface by measuring the
potential of the metal.
1. Cathodic protection
The principle of this method is to alter the electrode potential of the metallic structure so that they can lie in the immunity region. This is the region where the metal is in the stable state of the element and corrosion reactions are not possible. It is mostly used in steel structures in marine and under ground regions.
Two methods are used to apply the cathodic protection to a metal structure.
- Impressed
Current - This method is used for the
protection of pipelines and the hulls of ships in sea water. In this
method, an electric current is applied to the metal surface by use of DC
electrical circuit. The negative and positive terminal of the current
source is connected to the metal requiring protection and an auxiliary
anode respectively. The flow of electric current charges the structure
with electrons and changes the electrode potential in the negative
direction. This process continues till it reaches the immunity region. The
current flows from anode to cathode. Thus it protects the metal surface
from corrosion.
- Sacrificial Anode - This is especially used for ships, offshore oil and gas production platform etc. In this technique, the more reactive metal is used to alter the electrode potential and get the immunity region. Zinc is generally used as sacrificial anode. It generates the anodic dissolution current with more negative potential. The cathodic curve intersection is now at a more negative potential which is the immunity region. At this region, the corrosion rate of steel is negligible.
2. Corrosion Inhibitors
- According
to surface chemistry, the presence of foreign molecules affect the surface
reactions.
- Corrosion
processes are also a type of surface reactions. These can be controlled by
foreign compounds which are known as inhibitors.
- The inhibitors
get adsorbed on the reacting metal surface. It attaches directly to the
surface or adsorbs up to one molecular layer of the metal surface. This is
a well known method for controlling the corrosion.
- The
inhibitors can work in different ways; it may block the active sites of
corrosion and restrict the rate of anodic or cathodic process, or it may
increase the electrode potential etc.
- Hexylamine
or sodium benzoate are used as inhibitors for anodic reactions.
- Similarly,
oxidising agents like nitrite, chromate, red lead, amines, thio-urea etc
are also used as corrosion inhibitors.
Corrosion Resistance
Since corrosion deteriorate the physical and chemical properties of metals, therefore we always try to make corrosion resistance metal surfaces. There are various ways to make metals corrosion resistance. Few of corrosion control methods are listed below.
- Environmental Modifications
- Metal Selection
- Protective Coatings and plating
- Addition of inhibitors
- Corrosion Allowances
- Cathodic Protection
Corrosion
Testing
- Corrosion
testing is used to measure corrosion. This is done in corrosion testing
laboratories.
- These
are laboratories where experimental testing of materials is done for their
verification about corrosion according to various industry standards.
- Some
standards are used for this purpose like ASTM, ISO, NACE, or custom corrosion testing.
- Corrosion
testing also includes DOT test, electrochemical and immersion
test and heat transfer.
- Some
standardized methods are also used for testing of glass in different media
like acidic, basic or neutral.
- Testing
methods are done in specific conditions of environment. In the ISO method,
glass is put in the ionized water up to 60 minutes.
- This
solution is then titrated with HCl solution. Thus the amount of HCl for
neutralization is measured.
http://chemistry.tutorvista.com/physical-chemistry/corrosion.html
http://chemistry.tutorvista.com/physical-chemistry/effects-of-corrosion.html













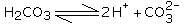







from your explanation, which the main factor of corrosion
BalasHapusI think the main factor of corrotion is O2 and H2O. In generally, metals are oxidize in contact with the air and covered by a layer of metallic oxide.
HapusHi Agungs, Whether corrosion can be prevented? How to prevent it?
BalasHapusThanks, you can do two methods for the protection of materials from corrosion.
Hapus1) Cathodic protection
2) Corrosion inhibitors.
Why is aluminum metal including reactive metal resistant to airborne corrosion?
BalasHapusThanks. In aluminium metal, the cover of aluminium oxide is very hard and thick which protects the metal surface against the later attack of the oxygen of the air. You must have seen a rusty iron hammer, which corrode by oxygen, but at a much slower rate than the burning wood.
HapusWhat distinguishes the speed of every metal?
BalasHapusWhy is tetanus disease often associated with rust? Is there really a connection?
BalasHapusCompare the length of time of corrosion, iron with copper?
BalasHapus